中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
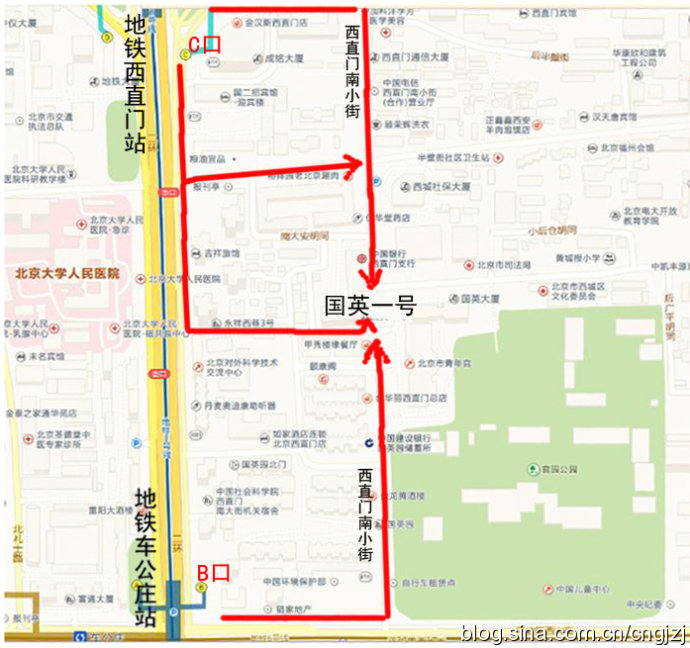
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
Regulations >>
Regulations
您现在的位置是:
首页 >>
Regulations >>
Regulations
2013年03月12日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
The stem cell preparations before the quality control and clinical research guiding principle (try out) "(draft)
The ministry of health of the People's Republic of China: www.moh.gov.cn
In order to standardize and promote stem cell therapy research in our country, ensure the safety of stem cell preparations, effective and controllable quality, at present already obtained the international knowledge of stem cell biology, stem cells and clinical application research progress, on the basis of product quality control technology, the ministry of health and state food and drug administration clinical stem cell research and application specification rectification work leading group to make the "stem cell preparations before the quality control and clinical research guidelines (trial)" (hereinafter referred to as "guidelines"), after repeated discussion changes after form the draft, and in March 2013 for advice throughout the country. Interpretation will now about the problem are as follows:
One, the background to the case
Predominantly stem cell therapy research at home and abroad in recent years has developed rapidly, in the treatment of degenerative disease, ischemic heart and cerebrovascular disease, joint disease, immune system disease, and transplant rejection, liver cirrhosis, diabetes, and other fields has launched a number of clinical trials. As a new treatment method, the safety of stem cell preparations need to have comprehensive study and evaluation of effectiveness, preparation quality directly decides the safety and efficacy of clinical treatment. Stem cell preparations need to pass before the preparation, quality control, clinical research and development studies (in vitro and in vivo tests) to the whole process of clinical trials. However, at present stage in our country is still a lack of stem cell preparations before the quality control and clinical research of related technical guidelines, difficult to correctly guide and regulate stem cell preparations related to research and development work.
Second, the basic principles
The "guiding principles" drafting is mainly based on the following principles:
1. Abide by the principles of scientific nature, risk control as the main consideration: in scientific aspects, the "guiding principles" as much as possible to integrate current stem cell biology, clinical research and the cell the new progress in the study of quality control, ensure to draft the basic train of thought is based on the understanding and analysis of the stem cell therapy related risk factors.
2. The reference cell treatment and biological products related guiding principle: in the complexity of the product, like stem cell preparations new biotechnology products, but are more complex. As cell product, its quality requirements should be in line with the WHO recommended for the cell substrate for production quality requirements. The "guiding principles" is mainly based on the guiding ideology, on the specific content, mainly refer to the state food and drug administration, 2003 edition of the human body cell therapy research and preparation quality control technical guiding principles, Chinese pharmacopoeia, the WHO, the European Union, the United States FDA and example of cells and biological products research and development related guidelines and references.
3. Reflect applicability principle: the "guiding principles" is a kind of preparation guidelines apply to all kinds of stem cells, so in draft the content of each chapter, mainly considering all kinds of stem cells preparations of common content, avoid features content and the specific and detailed requirements to influence the broad applicability of this guideline.
4. Have certain forward-looking: the guiding principles are formulated in order to steadily push forward China's stem cell research is one of the purpose of developing, so considering the domestic stem cell research at present technical level and, on the basis of actual situation, at the same time focus on development, so that the "guiding principles" in the future a period of time still has certain applicability.
Third, the main content
"Guiding principles" is divided into five parts, namely foreword, stem cells preparation of quality control, preparation of preclinical study, nouns explain and references. Introduction part briefly introduces the basic concept of stem cells and stem cells therapy, stem cell treatment at home and abroad research, stem cell research and development of the basic procedures and principles. The second and third part is the main part of this "guiding principles". Noun explanation is that this "guiding principles" in the most frequent noun or difficult to understand Chinese explanation, at the same time provide the corresponding English comparison. References listed the drafting of the "guiding principles" as the main reference literature.
Stem cells in the preparation of quality control is made up of four parts. The first part is "stem cell collection, separation and the establishment of the stem cells (department)", put forward the claim to the donor cells, general principle from the source to ensure that the stem cells no pathogenic microorganism pollution and obvious genetic risk factors; Secondly, this paper puts forward the preparation stage of quality control in the preparation of basic requirements. The second part is "the preparation of cell preparation", proposed to the stem cell culture medium, the quality control of trophoblastic cells and for the preparation of process management and validation requirements. The third part is "the stem cells in the preparation of inspection", this part puts forward the basic principles of the preparation for inspection, quality inspection and release of the main content, and all quality requirements; The fourth part is the quality of stem cell research, the main consideration is that, according to the development of stem cell science, stem cells preparation research and development personnel and quality control research professionals, should continuously expand the safety, efficacy and stability study of stem cells, constantly improve their ability to stem cell preparations, quality control technology.
Stem cell preparations of preclinical study consists of two parts, mainly in pre-clinical stage of research and evaluation on the effectiveness and safety of stem cell preparations.
Fourth, to other problems
(a) with stem cell research at home and abroad at present stage was still in the stage of exploration and development, technology is not mature enough, and our country lack technical guidelines for stem cell preparations before, so this guiding principle of short duration as the pilot files released, will in future according to implementation timely for further modification, enrich and perfect.
(2) research on stem cells, quality inspection and quality of different consideration: stem cell preparations quality test listed in the content, mainly based on the internationally recognized as one of the basic requirements of quality of stem cell preparations, and at present, the cell quality inspection agencies to carry out professional content, both the scientific nature and operability, so should as a general inspection work must be completed; And listed in the stem cell preparations quality research content, main is to encourage research and development personnel and professional quality inspection organizations according to the present related stem cell quality deep scientific cognitive difficulties or problems in study, to create a new test for safety, efficacy and stability of the stem cell technology, at present temporarily don't consider it as a regular quality inspection content.
Related links: ask for the stem cell clinical trials management approach (trial) "and other documents on the letter