中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
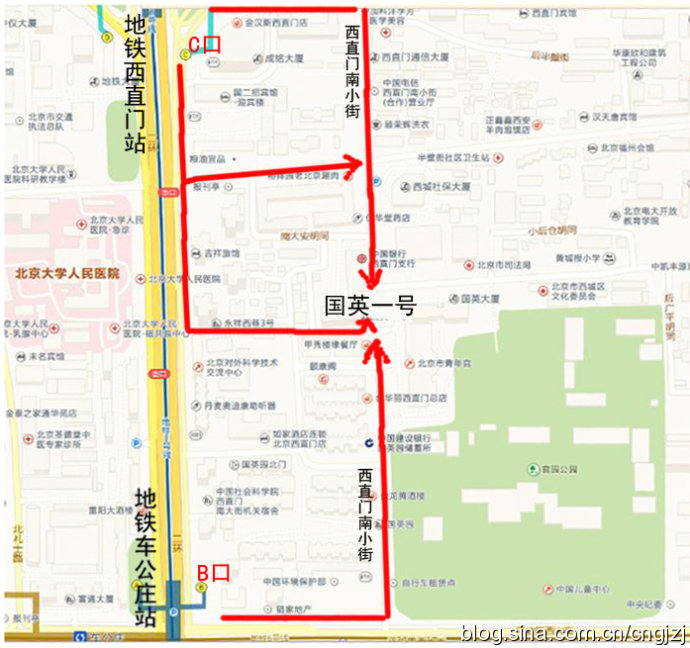
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
R&Dplatform >>
R&Dplatform
您现在的位置是:
首页 >>
R&Dplatform >>
R&Dplatform
2014年07月14日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
Research and development of new drug of TCM needs to reduce the risk of policy
Beijing business today
In recent years, the difficulty of examination and approval of new drug of TCM is increasingly prominent. Said war Carrie, director of the Beijing institute of traditional Chinese medicine scientific research projects, with a new drug research and development, the first thing to consider several factors, including project feasibility, the review pass rate, clinical utilization, market prospect, research and development cycle is long, technology risk, policy risk increase year by year is encountered several big obstacle for examination and approval of new drugs.
From the drug research and development to the market for at least ten years
, the difficulty of examination and approval of new drug of TCM more prominent in recent years, there are, according to the research and development of new drug application can get approval documents clinical research probability is very low, and the clinical application can be accomplished by approved clinical test and get a new drug certificate is about 30%, that is to say ten applications for new drug research and development and finally also can one or two into the clinical application, the passing rate is extremely low.
Examination and approval of new drug of TCM why so difficult? The Beijing institute of traditional Chinese medicine focuses on the study of new drugs and medical institutions in the new preparations of Chinese medicine, Carrie's told Beijing business newspaper reporters, for new drug research and development institutions, first requires abundant funds, advanced technology, also has a long fight. At present there are two main force of new drug of TCM, is a national scientific research units, one is the strength of large enterprises, small and medium-sized enterprises and small research institutions are difficult to support. In terms of Beijing institute of traditional Chinese medicine (TCM), the entrusted to accept the new drug research and development in recent years are numbered.
"A new drug research and development from the application materials submitted to the state food and drug supervision and administration bureau, the drug approval center staff to open the file, the deadline for seven or eight months are normal, before filing varieties backlog didn't get a reply. There are even two or three years in general, from drug research to the clinical, to the market for at least ten years, also not necessarily can be successful." Carrie said, research and development of new drug approvals and cycle is very long, from the project demonstration, project, application of basic research, clinical licensing, application of clinical trials, production license, this a series of program operation, KuaiZhe five years rarely, common ManZhe decade eight years.
"Schedule" in the face of the policy risk
Beijing commercial daily reporter learned that, for a new drug research and development, the first thing to consider several factors, including project feasibility, the review pass rate, clinical utilization, market prospects, with particular emphasis on optimal effectiveness and safety of new drugs. "In the past research and development of new drugs is high risk, high investment, high return, now may be the high risk and high investment, but not necessarily high returns. Loose careless project, the research will lead to the deficiency of new drug research and development, an incorrect one false move may lose the game, technical risk is great. In addition, the policy risk increase year by year, is also one of the factors of examination and approval of new drug of TCM is difficult." Battle of related new drugs policy gradually Carrie rein drug safety risk agreed, but also hope that relevant policy to consider the difficulty of the enterprise development fast strain. Such as medicinal herbs extract active ingredients, purity, impurity content requirements are drug review policy changes in the factors, such as can constantly adjust, once can't satisfy the new requirements, the front research and development will be disqualified. Due to the particularity and the complexity of drug development, cycle is very long, even if the authorities have to solicit comments from society, but also tend to make research and development unit off guard.
"So now the new drug research institutions often play 'schedule, the current policy requirements need to be, there is no requirement of r&d is thought of as much as possible, don't do only 60 minutes, mark according to 80 points, 100 points in the process of research and development, in case of policy adjustments to keep up with changes." Carrie said, running this policy reversed transmission research and development unit of the phenomenon, make research and development of new drug of TCM more unpredictable factors.
The excessive development sparked a rising tide lifts all boats
Are constantly improve the review requirements of drugs is good? War Carrie's concern, research and development unit, "nc" companies are doing, thinking beyond existing requirements, it is easy to cause a rising tide lifts all boats. See the market moving, government policy adjustment followed, and the policy adjustment is fully in line with the situation and the development characteristics of Chinese traditional medicine? It's not.
Take Chinese traditional medicine raw materials, for example, he said, as we all know, medicine effective component by origin, growth cycle, collecting time, processing, storage, and many other factors, is not very stable, some enterprises in order to meet the policy requirements, approval documents do medicine constant uniform, is asking for the origin of a product, and good local herbalist about planting, picking time, two or three consecutive batches in stock, then the raw material blending, then a batch of production, two, three, to guarantee the stability of active ingredients, this will make the Chinese medicine greatly increased the cost of production. Battle in Carrie's point of view, is not can't improve drug review requirement, but should follow the current situation and the development rule of the market, according to the characteristics of the traditional Chinese medicine, to develop a drug quality can be ensured without excessive increase of the cost of the policy, the direction and guidance advice, rather than to use the evaluation model of western medicine drugs, the stricter the better, reversed transmission market followed the policy guidance.
Beijing commercial daily reporter In the summer of shanshan