中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
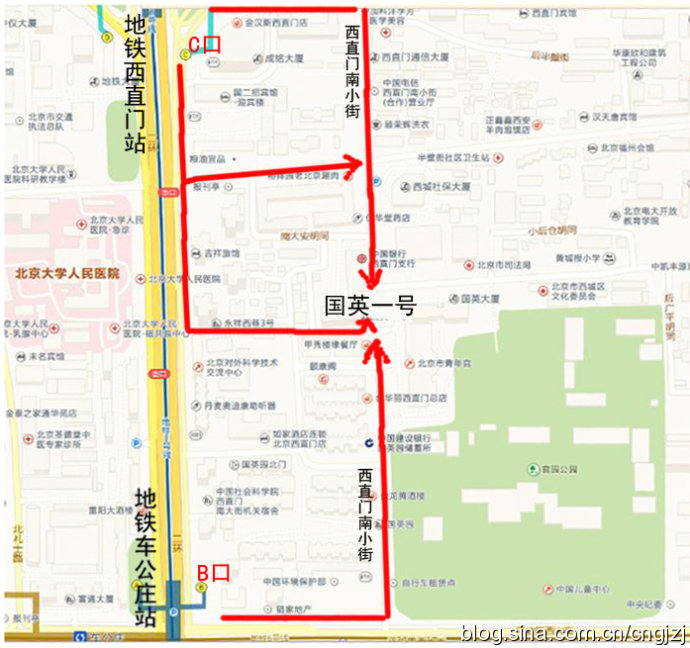
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
R&Dplatform >>
R&Dplatform
您现在的位置是:
首页 >>
R&Dplatform >>
R&Dplatform
2014年12月22日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
Traditional Chinese medicine "quintessence" can't be wasted
08:48 on December 8, 2014
Business club on December 8
Chinese medicine is the treasure of the Chinese nation, however, as the birthplace of Chinese medicine, but not a monopoly in the field of traditional Chinese medicine industry in our country.
In 6, the fifth China modern traditional Chinese medicine industry development on the BBS, the experts, the herbal medicine market has more than $60 billion in total, in which our country share less than 10%, a lot of proprietary Chinese medicine resources of intellectual property rights was also criticised by foreign invasion. They called for traditional Chinese medicine resources can't be wasted.
Do not take the patents, more than 900 Chinese medicine prescription by foreigners "steal"
The Japanese in treatise on febrile disease, based on synopsis "and so on more than 210 prescriptions, building up to more than 200 Chinese prescription medicine. On the basis of traditional Chinese medicine research and development of "kyushin pills", annual sales of more than $100 million. China's invention of artemisinin and patented by the germans, loss of $200 million to $300 million each year in China. South koreans to tongrentang (21.76, 0.02, 0.09%) bezoar sedative bolus formula, change the dosage form in 19 countries and international organizations to apply for a patent in the world, with annual sales of $70 million. In February, the German bayer Chinese herbal medicine industry layout, RMB 3.6 billion annexation dian hong pharmaceutical...
According to statistics, China has more than 900 kinds of Chinese herbal medicine by foreign companies in overseas project first to apply for patent. And so far, few Chinese traditional medicine can through the FDA (us food and drug administration) into the west hospital prescription market.
"In recent years, domestic OTC (over-the-counter) drug firms struggling, slow growth, thus holding a heavy gold to buy foreign companies, will become the OTC drug firms have a choice." Professional committee of China association of traditional Chinese medicine (TCM) and protection of Chinese innovation Liu Yanhuai said, if not speed up the traditional Chinese medicine intellectual property protection, our fathers of the future will be to eat "prescription" and deliver the royalties to foreigners.
Is back in the patent race, in December last year, the state administration of traditional Chinese medicine was established the "world traditional medicine patent database", this is the world's most complete included natural medicine patent database.
"Based on the database, data mining methods for traditional Chinese medicine formula design are available, and this provides a high efficiency low cost development way of compound drugs." Liu Yanhuai also suggested that the combination of traditional Chinese medicine has a core value in the medical application for patent, Chinese herbal medicine on a geographical indication protection of new varieties of Chinese herbal medicine to provide new plant varieties protection, attaches great importance to the scientific and technological innovation.
Pharmaceutical test data protection system for reference, to expand protection areas
Chinese medicine not only need to patent protection, also need to develop other system level protection.
Such as the United States in the 1984 years ago, has not yet been submitted by the applicant to disclose the drug safety and effectiveness of data taken limited commercial secret protection system, in order to prevent unfair competition. But these data acquisition, requires a lot of time and money, generics are easy to ride, no effective to protect the interests of the new drug developer. In addition, some drug approval after the basic research of research and development and the process is longer, make effective business patent protection period shorten, some drugs also cannot get patent protection because of the loss of novelty.
Government and drug regulatory administrative department, developed countries have introduced measures, implementation of the "data protection" system, in parallel with the patent protection system, effectively promote the pharmaceutical innovation. "Under the current system in our country, traditional Chinese medicine (TCM) clinical endorsed a long time after 2000, approved by less than 10% of new drug of TCM, more than 80% of the traditional Chinese medicine (TCM) on the market at present is the old varieties." Administration of state food and drug supervision and administration of traditional Chinese medicine (TCM) varieties protection review committee office director Chen Guangyao said. Due to the particularity of traditional Chinese medicine (TCM), a kind of traditional Chinese medicine new medicine often did not enter the harvest in listing 10 years later, before that, the patent period basic is over.
He suggested that our country can draw lessons from pharmaceutical test data protection system, improve the system of protection of TCM variety, improved varieties of traditional Chinese medicines protection system should not only protect the new drugs, but should also protect finished the second development of older drugs, with 5-10 years advisable protect time, both traditional Chinese medicine (TCM) double attribute of the inheritance and innovation, the formation of modern classic varieties in the field of traditional Chinese medicine (TCM).
The world's largest Chinese herbal DNA barcode database, is about to debut
"Find every species of right of traditional Chinese medicine, to create a gene barcode database. At present, Chinese pharmacopoeia all medicinal genetic barcode have been established, immediately out of the" Chinese pharmacopoeia "DNA sequences, to create the world's largest Chinese herbal medicine DNA barcode database and identification platform." China institute of traditional Chinese medicine academy of sciences, said Chen sl is introduced, the establishment of the system is expected to significantly improve the safety and efficacy of Chinese traditional medicine.
Breakthrough exciting, basic work, the mode of innovation breakthrough is still needed. Executive deputy director of the institute of China academy of traditional Chinese medicine basic theory Hu Jingqing found during inspection at the Pasteur institute in France, the institute was founded in 120, received 10 Nobel Prize, also created income of 2.1 billion yuan in 2009, the basic research with the domestic situation is completely different. "Pasteur was a biologist, but very pay attention to technology application." Hu Jingqing said, in order to meet the demand of industrial users as the core, the Pasteur institute has helped set up 14 emerging companies, including France's sanofi-aventis group, etc. He suggested that our country enterprises and research institutions of traditional Chinese medicine, can draw lessons from the Pasteur institute, focus on market demand to do research.
Positive boost industry progress and cooperation
Since 2010, BBS platform structures, enterprise development policy advice for traditional Chinese medicine, enterprise management, marketing services, contributed to the Chinese medicine industry technology innovation strategic alliance for national alliance, in order to further ensure the standardized planting the Chinese herbal medicine, hubei province, new product development contribution strength, etc.
BBS has become an important force to boost the progress of Chinese herbal medicine industry, also contributed to the communication and cooperation between enterprises. Such as, led to the strong brand co., LTD and kyushu tong (20.18, 0.57, 2.75%) medical group cooperation, contributed to the enshi, YingShan, macheng, liaocheng six Chinese medicinal materials, such as standardization of base construction, strongly improve the hubei Chinese drugs in the national market influence and voice.
Successive BBS industry consensus
The first: proprietary Chinese medicine modernization cannot leave a GAP (Chinese herbal medicine production specification); Traditional Chinese medicine industry in the future competition, must be the competition of resources.
The second: traditional Chinese medicinal materials quality is Paramount, standardization, standardization planting is the precondition of industrialized production.
The third: drug development from industry leading to clinically oriented; The effectiveness of traditional Chinese medicine to make significant progress, security is more important.
The fourth: drug innovation need to dig big data; Use data to ensure drug quality herbs species.
(article source: hubei daily)