中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
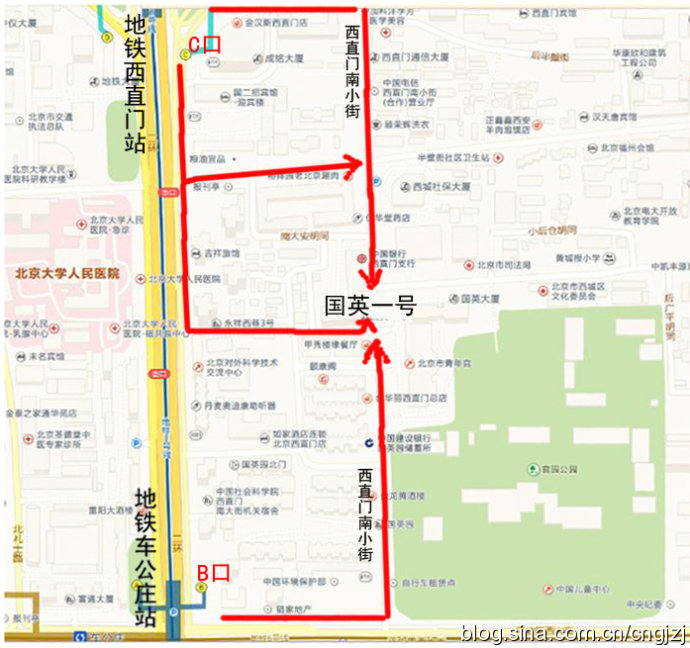
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
Regulations >>
Regulations
您现在的位置是:
首页 >>
Regulations >>
Regulations
2015年12月14日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
Carry out the work of stem-cell research institutions for the record about the notice
The national health and family planning commission of the People's Republic of China
The 2015-12-07
Countries who do science letter [2015] no. 1071
Provinces, autonomous regions and municipalities directly under the central government health family planning, food and drug supervision bureau, the xinjiang production and construction corps health, food and drug supervision bureau.
To promote the "stem cell clinical research management approach (trial)" and "stem cell preparations before the quality control and clinical research guiding principles (try out)" implementation, strengthen the management of clinical research institute for stem cell, earnestly implement the main responsibility of stem cells in clinical research, to ensure the qualified medical institutions specification to develop stem cell clinical research, promoting the healthy development of stem cell clinical research state health and family planning commission and food and drug supervision administration will accelerate the work of the stem cell clinical research institutions for the record. The concrete issues notice is as follows:
A, declare conditions
Declaration agency shall conform to the "stem cell clinical research management approach (trial)" article 7 requirements.
Second, the declaration material
(a) conform to the "stem cell clinical research management approach (trial)" annex 1. Specific material provided is as follows:
1. The license certificate copy of practice of a medical institution;
2. The drug clinical trial institution qualification certificates);
3. The stem cell clinical research organization management system (frame) and the departments of responsibility;
4. The stem cell clinical research management mainly responsible, quality authorized person qualification, as well as the relevant personnel training;
5. Organization of academic committee and ethics committees and their working system and standard operating norms;
6. Stem cells in the preparation of standard operating procedures and facilities, personnel conditions;
7. Stem cell clinical research quality management and risk control procedures and related documents (including the quality management manual, clinical research work procedures, standard operation specification and test records, etc.);
8. Stem cell clinical research audit system, internal audit and external audit, internal audit personnel qualification;
9. Stem cell quality evaluation standard and testing facilities;
10. Research on stem cells to prevent clinical risk management mechanism and treatment measures of adverse reactions, adverse events;
11. Other relevant information.
(2) host or as a major bear the national or provincial units of stem cell research subject and related proof materials.
Third, according to the procedures
(1) of the provinces, autonomous regions and municipalities directly under the two committees bureau in accordance with the "stem cell clinical research management approach (trial)" and "stem cell preparations before the quality control and clinical research guiding principles (try out)" for stem cells in this region filing work of clinical research institutions.
(2) the declaration agency to declare the material (A4 paper double-sided printing and binding) two copies and electronic disc 2, submitted to the provincial (autonomous regions and municipalities), health and family planning commission of scientific research management department by provinces, autonomous regions and municipalities directly under the health and family planning commission jointly with the food and drug supervision bureau approval.
(3) the provinces (autonomous regions and municipalities) health and family planning commission will after the audit committees at the provincial level administration of filing materials a type 1 a and electronic cd-rom, on December 10, 2015 the state stem cell clinical research expert committee secretariat (address: Beijing dongcheng district wide channel home 10th floor, room 1013, tower 2, zip code: 100022, contact phone number: 010-62115986).
(4) the national stem cell clinical research expert committee authorized by state health and family planning commission and food and drug supervision administration, for technical audit declaration record filing materials. The national health and family planning commission and the food and drug supervision administration of the record-keeping administration for the public. Public institutions, there is no objection to register put on record information system in medical research on stem cells clinical research institutions to register for the record. Stem cells for clinical research project should be implemented in the already registered organization.
Contact: national health KeJiaoSi state family planning commission Yin Xuke, Wang Jinqian
Telephone: 010-68792955
Fax: 010-68792955
Contact person: the food and drug supervision administration of drug registration department Chang Weigong
Telephone: 010-88330713
Fax: 010-68316572
General office of national health and family planning commission The food and drug supervision administration office
On December 1, 2015