中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
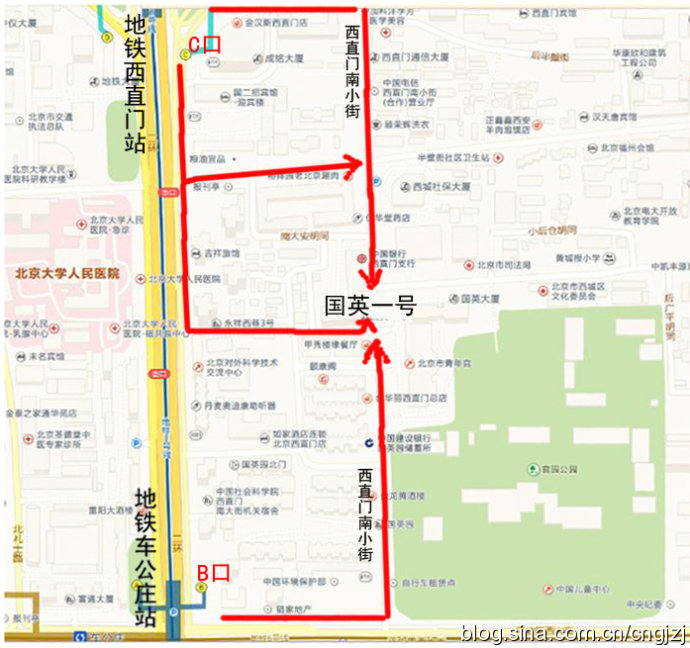
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
R&Dplatform >>
R&Dplatform
您现在的位置是:
首页 >>
R&Dplatform >>
R&Dplatform
2016年03月09日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
Biological similar medicine VS chemical generics
The 2016-03-09 10:05:44
source: medicine economic news author: David shou-yeh wong
Biological medicine and chemical generics which all have a common goal of saving lives, and belong to a generic category, but significant difference. Compared with the chemical of generic drugs, biological medicine mainly has the characteristics of "projects" : namely high technical threshold is high, the investment threshold.
It is generally believed that similar biological medicine research and development usually requires 8 ~ 10 years, 3 ~ 5 years than chemical medicine generics much longer. One of the world's biggest generics firm, famous multinational drug companies owned by sandoz think a kind of typical chemical generic generic cost $2 million to $3 million, and biological drugs like that number is as high as $075 million to $250 million, both differ one hundred times.
Although r&d cost time and money in different reports vary, but there is no dispute is that creatures like medicine more than chemical needed for generic drugs, investment cost is higher. And the different, is due to the many different of the two kinds of medicine, this paper tries to explore the difference between both.
[PK article]
The original biological medicine, biology, medicine, chemical similar comprehensive comparison of generic drugs
To understand the biological medicine and chemical similar generic differences, first of all need to know the difference between the original biological medicine and chemical medicine, the author summarizes the difference in tabular form system. List the author also summarizes the biological similar to the primary difference between medicine and chemical generics.
The product itself PK
Small molecule chemical medicine is usually chemical synthesis, and biological medicine is usually biological macromolecule composite. Both in the source of different, as a direct result of both in the structure, composition, production method and equipment, intellectual property, formula, save method, dose, regulatory mode and marketing mode are different.
Compared with the synthesis of small molecule chemical medicine, biological medicine in molecular size is one hundred times bigger to thousands of times. Antibody drugs such as molecular weight of 150000 daltons, molecular weight and chemical medicine is usually less than 1000 Dalton. Some reported compared the size of a small molecule chemical medicine to a bike, while the size of the biological medicine is equivalent to an aircraft.
In fact, the difference between the two is not only the difference of molecular size, more important is the molecular structure of biological medicine is much more complicated than the chemical medicine. Protein drugs, for example, a primary structure (amino acid sequence), secondary structure (such as alpha helix, folding, etc.) and the tertiary structure is more complex. Some biological drugs, stabilizing the tertiary structure of protein intermolecular combination can also form the quaternary structure.
More complicated is that after biosynthesis of these structures is often a posttranslational modification (PTM), including glycosylation, phosphorylation, etc. And these modification, different batches of biological medicine will also vary. These changes in biological medicine biological activity can be very critical.
Biological medicine and chemical medicine is another important difference between their immunogenicity, almost all therapeutic protein in the human body to produce antibodies. They will pass and endogenous factors and reduce the energy and even induce serious side effects.
Although a lot of differences between biological medicine and chemical medicine, but the author thinks that the core and the most important difference between two points, it is also for biological medicine must also have at least two conditions: biology and macromolecular synthesis. Some small molecule chemical medicine can biosynthesis methods; Now the chemical synthetic peptide synthesis technology can be as high as more than 100 amino acids, molecular weight can be tens of thousands of Dalton, but even such a large polypeptide protein (or small) is not biological medicine.
The production process PK
Due to the complexity of biological medicine has greater molecular weight and structure, characterization of biological medicine is facing great challenges. Although with the progress of modern science and technology, the analysis of the characterization of biological medicine technology is more and more advanced, but due to the characteristics of the above, even if the world may have the most advanced instruments and equipment used, also it is not possible to structural features such as biological medicine fully express clearly. These features also destined to biological medicines can't completely and similar drugs exactly the same, even if is the same company in the production of the same kind of biological medicine, different batch will have difference, even the same batch, in the process of storage, circulation, and biological drugs, especially protein drugs) structure and activity is inevitably changing.
Similar to biological medicine producers, due to various reasons such as the protection of intellectual property rights, the original drug production process adopted by the company and even cell lines are not clear, it's more cause biological medicines and similar drugs won't be the same.
In addition, the biological medicine production and circulation process is more complex, demand is higher, there are many steps, cell culture conditions (temperature and nutrition), product processing, purification, storage and packing and so on each link will affect the production of products, the tiny differences in the whole process are likely to final product quality, purity, biological features and clinical effect of larger impact. Is due to the above various reasons, although the chemical generics in English is a generic drug, but a similar drug is not a biogeneric, but biosimilar, because biological medicine can only be similar with the original drugs "similar" (similar), impossible.
For traditional small molecule chemical medicine, however, usually have very sure and stable chemical structure, the analysis of the existing methods (such as infrared, nuclear magnetic resonance (NMR) and X-ray diffraction, mass spectrometry, etc.) is enough to fully understand its chemical structure.
Price cut space PK
So, in general, biological medicine production to the requirement of the production conditions than chemical medicine, of course the production cost is also higher. And preclinical and clinical stages of biological medicine research and development costs are higher, because regulators, particularly in Europe and the United States require similar drug producers to provide enough clinical data that biological medicines and similar drugs have the same clinical curative effect, leading to similar biological medicine listed on the approved generic costs tend to be in front of the chemical medicine is one hundred times.
For similar high generic drug cost and production cost, the general biological medicines and similar drugs, compared to only 10% ~ 30%, while chemical generics can be as high as 80% or more (this especially for India's manufacturing chemical generics). Therefore, chemical drugs once the patent expires, is generic onslaught, sales will drop dramatically, and chemical generics will soon take market (this, after the "who" Lipitor Lipitor evidenced); While the biological original drugs in the patent expires, sales less affected by generic drugs.
Regulatory policy PK
Biological medicine and chemical similar generic difference is also reflected in the post-market supervision. Because of the same chemical generics and branded drug structure, and the structure is simple, the European and American regulators allow independent pharmacists with chemical generic drugs to replace the original drugs (i.e., the automatic replacement policy, or alternative, interchangeability), without having to notify doctors prescribe. And similar to biological medicine, in the eu, the laws and regulations are not allowed to automatically replace clear requirements. For now the FDA has officially released about biological medicine guidelines, similar biosimilar and can be divided into two categories: one is similar to the original biological medicine height of ordinary biosimilar; The second is interchangeable biosimilar.
Can automatically replace similar drugs, namely the interchangeable biosimilar, more strict than ordinary biology of the medicine. Only so far, the FDA has approved a similar drugs, and does not allow for automatic replacement. The author predicts that at least 3 ~ 5 years in the future is difficult to have can automatically replace the biological medicine approved listing in the United States.
For the automatic replacement policy, the author thinks that it is necessary to say a few words: more interchangeable if translated into automatic replacement can cause ambiguity and interchangeable in fact not really "automatic" replace automation system, but according to the regulations of the United States, pharmacy or hospital pharmacists can according to your own knowledge and experience to judge whether prescription drugs can be replaced. Biological similar medicine "only child" in the United States - March 2015 approved the sandoz Zarxio (filgrastim - SNDZ), for example, if an American doctor's prescription is into Neupogen, chemist cannot presumptuously use Zarxio to replace, because Zarxio not interchangeable biosimilar. And if Zarxio is interchangeable biosimilar, pharmacists can not informing doctors prescribe Zarxio will be used to replace Neupogen. So far as mentioned above, the FDA has yet to approve any kind of interchangeable biosimilar.
Due to the highly complexity of biological medicine, biological medicine can do it with the original drugs similar height similar already very not easy, therefore, to do with the original drugs as replaceable similar biological medicine, difficulty is not common. To achieve what kind of standard is interchangeable biosimilar? The FDA has not yet issued clear guidelines that produce such guidelines is not an easy thing.
Article [market]
In reserves, the world's research in our country, in the next 10 ~ 15 years is the golden period
Compared with chemical medicine, more complex and usually more expensive biological medicine to enter the market will undoubtedly face a bigger challenge, especially for low levels of the developing countries.
At present in China, the local biological medicine (most is the so-called the first wave of similar drugs, such as interferon, growth factors, etc.) in a smaller proportion of the total drug market, and in Europe and the United States, approved in recent years, the number of innovative biological medicine basic accounts for about thirty percent of the total approved drugs (in the United States, if you count the FDA CBER batch of biological medicine, the proportion higher).
Biological medicine prices generally higher, but also promote biological medicine market share continues to rise quickly. Globally, the biological medicine is in its infancy, basic similar biological similar drug is share of the market are small, so to speak. However, the industry is generally believed that the next 10 ~ 15 years is golden development of biological similar drugs. According to the forecast IMS Health, to 2020, the similar biological medicine is expected to reach $25 billion in annual sales, accounts for about 10% of the biological medicine market share.
In the face of such a big cake, as generic power in our country, the conditional domestic drug firms to launch natural biological similar drugs, no condition is also trying to. So now, our country in the research of biological medicine similar number (mainly the preclinical phase) is more than the United States, to become the world's first (according to Thomson Reuters data).
So many similar drugs, if only rely on the Chinese market, obviously can't digest, into Europe and the United States international markets that will be the inevitable choice of domestic some powerful drug firms. But, especially in Europe and America the biggest global drug market for the United States to the biological medicine similar regulation is very strict, so the Chinese drug firms to counsel. Especially the most difficult is the highest gold content of monoclonal antibodies similar biological medicine, has so far not a single biological similar drug resistance from China began clinical trials (BLA) approved in the United States, throughout the greater China region only Taiwan xi kang (zhenhua) scored in Europe, the first clinical trials in Europe began to own rituximab similar biological medicine (namely) of rituxan JHL1101.