中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
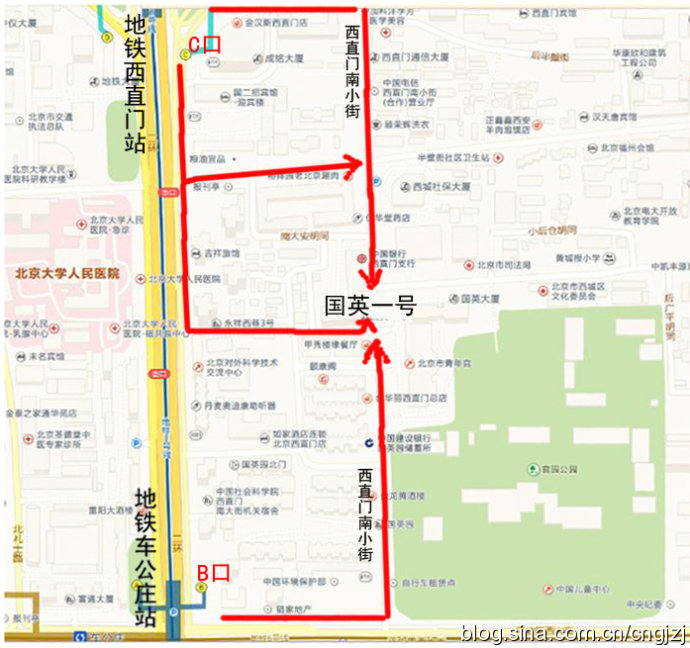
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
Regulations >>
Regulations
您现在的位置是:
首页 >>
Regulations >>
Regulations
2017年07月06日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
To reform and improve the supply guarantee mechanism for shortage of medicines
Release date: 2017-06-28 national health administration [2017] no. 37
The people's government of the provinces, autonomous regions and municipalities directly under the central government, the xinjiang production and construction corps:
For implementation of national health and health conference spirit and the "healthy China 2030" planning outline ", "" much starker choices-and graver consequences-in" deepening medical and health system reform plan ", "" much starker choices-and graver consequences-in" health and health plan ", "the general office of the state council on further reform and improve the pharmaceutical production circulation use policy several opinions, reform and improve the shortage of drug supply security mechanism, to better meet the needs of the people's health and clinical rational drug use, and enhance people's sense, agreed to by the state council, put forward the following suggestion on now.
I. general requirements
(1) guiding ideology.
Fully implement the party's 18 large and 18 sixth plenary session of 3, 4, 5, spirit, carry out general secretary xi series of important speech spirit and governing the new strategy, new ideas, new ideas as a whole to promote "five one" the overall layout and coordination to promote "comprehensive" four strategic layout, always adhere to seek improvement in stability work tone, firmly set up and implement the new concept of development, adhere to the work under the new situation of health and health policy, by the people's health as the center, in order to satisfy the demands of clinical rational drug use as the guide, we will deepen reform of the medical industry supply side structural and in accordance with the "classification, classification management, in consultation with linkage, security supply" principle, establish and improve the shortage of drug supply security system and mechanism, improve the efficiency of factor allocation and effective supply ability, for comprehensively deepen medical health system reform, promote the health of China construction to provide strong support.
(ii) main objectives.
By the end of 2017, set up the mechanism of drug shortage of information collection and summary analysis, to refine the list of shortages drug monitoring and early warning and management system, and built a preliminary application based on large data of national drug supply security integrated management platform and shortage of drug monitoring and early warning information system, perfect department in consultation with the linkage mechanism, a preliminary establish national, provincial, city and county level monitoring and early warning mechanism and the national, provincial level two coping mechanism.
By 2020, realize the shortage of drug supply security comprehensive management and monitoring early warning and the sharing of information resources sharing, establish a mature and stable drug shortage of real-time monitoring and warning and grading system, build the shortage pharmaceutical information collection, summary analysis, department coordination, graded response, industry leading "five one" work pattern, forming a shortage of drug supply security system with Chinese characteristics.
Ii. Key task measures
(1) to improve the monitoring and early warning and list management system of shortage drugs.
1. Reasonable layout monitoring post. Layout in the present each province on the basis of not less than 15 monitoring sentinel, based on a national drug supply security integrated management platform, provincial drug centralized purchasing platform (public resources trading platform), regional population health information platform, industry association and its member units, gradually expand the scope of monitoring, unicom register pharmaceutical research, procurement, production circulation use such as the key link, gradually realizing a complete coverage of drug shortage of information monitoring.
2. Establish and improve the list management system of shortage drugs. Comprehensive analysis on the disease changes, key crowd clinical medication requirements, emergency safeguard demand, production of active pharmaceutical ingredients and drug approval, etc., reasonable definition standards necessary for clinical drug shortage, build national and provincial levels shortage drug inventory management system. According to the situation of shortage, shortage and scope of influence, timely launch the national or provincial response mechanism and release relevant information regularly. Integration of multi-source monitoring data information, step-by-step implementation departments shortage drug listing information linkage, open government, medical institutions, enterprises, social organizations and relevant data, sharing, common channel. The organization carries out the clinical comprehensive evaluation of the drug in the list, keeps the list constantly, and realizes the dynamic management of the shortage drug inventory.
3. Enhanced comprehensive monitoring and early warning. We will implement the monthly zero reporting system for monitoring information of shortages, improve monitoring indicators and improve monitoring efficiency. To guide local departments at various levels in timely analysis, processing and reporting of monitoring information, and establishing a four-level monitoring network system and early warning system in countries, provinces, cities and counties. Construction based on the shortage of big data applications drug monitoring and early warning information system, strengthen the shortage of drug research and development, production, distribution and usage of comprehensive evaluation, enhance the efficiency of the information monitoring, analysis and processing and gradual shortage of drug information whole process dynamic perception, the policy of the early warning monitoring and evaluation, to prevent such as intelligent application.
(2) establish a mechanism for the coordinated response of the supply guarantee for shortage of drugs.
4. By the national health and family planning commission, the national development and reform commission, ministry of industry and information technology, ministry of finance, human resources and social security ministry, the ministry of commerce, state-owned assets supervision and administration commission of the state council, state administration for industry and commerce, food and drug supervision administration and so on national drug supply shortage of security work in consultation with the linkage mechanism, policy of outstanding interdisciplinary and multi-sectoral overall, coordinated and effective linkage, clear division of work rules and task.
5. A national level key around national drug shortage list varieties, organization in production of active pharmaceutical ingredients and drug supply shortage of support capability assessment, shortage of research to perfect the drug supply security major policy and system, solve the problem of shortages across the province and coordination; Fully relying on and integration of existing resources, accelerate the construction of drug clinical comprehensive evaluation system, promote the drug purchase unified coding standard application, shortage of overall good drug monitoring and early warning information system as well as national and provincial levels drugs multi-source information collection and supply of the construction of business application platform together.
6. The provincial health and family planning department coordinated the relevant departments to establish a corresponding mechanism for consultation and consultation, comprehensively assessed drug shortage information and response Suggestions in jurisdictions, and comprehensively addressed the problem of local shortage. We will strengthen the three-level monitoring of provinces, cities and counties, timely analyze, process and report the shortage of information, and enhance the comprehensive response capacity.
(iii) the implementation of the shortage of medicines to ensure the classification of the precise application.
7. Implementation of fixed-point production. Comprehensive clinical required, small dosage or market price is low and the shortage of power enterprise production factors, selecting the fixed-point production varieties, through the government pricing, price negotiations, market set a variety of ways, such as to determine the unified purchase price, bidding to determine fixed-point production enterprise, the net purchase directly, guarantee reasonable supply area.
Coordinate emergency production and import. In the case of the changes in the related standards of drugs and the improvement of the certification, the company has been able to coordinate the emergency production of the enterprises and accelerate the importation of the qualified enterprises. We support the upgrading of related enterprises' technological upgrading, and support the centralized production base of small variety drugs with strong comprehensive strength and small variety drug approval Numbers. We will support the active participation of all parties in pushing soes to fulfill their social responsibilities and ensure sustainable and stable supply.
9. Strengthen the supply and demand of supply and demand, consultation and adjustment. Pay attention to grasp the pharmaceutical production circulation enterprises and actual inventory of medical and health institutions to promote clinical demand side and supply side to strengthen production circulation, medical and health institutions in drug shortage, timely consultation dispensing drugs, avoid sharing of information between supply and demand led to the shortage.
10. Improve the shortage of drug reserves. According to the actual needs of the clinical practice, we will screen the shortage of drug reserve varieties (including the raw materials), reasonably determine the quantity of the reserves, arrange the collection funds, and ensure the timely and effective call of the drug reserve. The central and local levels of the drug reserve are established, and the central medical reserve is mainly used for the use of uncertain drugs.
11. Cracking down on illegal and illegal activities. Strengthen the material supply of goods, business inventories and market transaction behavior, such as monitoring, comprehensive assessments MiaoTouXing issues and trends, the price rise significantly in the production of active pharmaceutical ingredients and drug distribution enterprises pay close attention to, when it is necessary to carry out the cost price of special investigation. We will strengthen the market supervision of drugs and raw materials, and investigate and punish illegal behaviors such as price fixing and monopoly in accordance with the law. We will increase penalties and maintain market order. Study and establish the drug shortage of monopoly API price behavior guide, build system of faithless operator blacklist, the shortage of repeatedly check and make active pharmaceutical ingredients and drug products is relevant to the monopoly operator, in accordance with the prohibition against the measures in the drug industry. Strictly implement the drug procurement integrity record and market clearing system.
12. Sound policies for the use of rare diseases. Research to establish the common rare diseases medicine database, through the national science and technology major projects such as national r&d project support enterprises and scientific research units research and development innovation, will be eligible, be badly in need of a rare disease clinical application listed in the priority list of r&d, to improve and implement policies on rare diseases medicine priority review for examination and approval.
3. Organization guarantee
(1) strengthening of organizational leadership. We should give full play to the institutional and institutional advantages, strengthen the construction of the policy coordination mechanism for drug supply and guarantee, and build a system of governance that is dominated by the government, participation of enterprises, social cooperation, and a shortage of medicines for all. National health and family planning commission jointly with relevant departments to pay special attention to organize the implementation, strengthen information communication and monitoring and early warning, to strengthen the comprehensive coordination and supervision assessment, timely report to supervise the evaluation results, give priority to promote shortage varieties of essential drugs list, id, price, purchase, equipped with use of uniform policy, perfecting the systems and mechanisms. The local people's governments at or above the county level must strengthen the shortage of drug supply to ensure the leadership responsibility, the shortage of drug supply protection into the government work performance appraisal system, strengthen supervision and oversee incentive accountability, make sure to obtain actual effect. Relevant departments should refine policy measures, clarify job requirements and improve the long-term working mechanism. The local government and relevant departments that have failed to implement the measures shall make timely and relevant discussions, causing serious consequences, and shall be held accountable according to law. We will encourage pilot demonstrations in areas where the basic conditions are good and the work is highly motivated, and explore the typical practices and effective models that can be replicated and promoted.
(2) increase policy support. Research and development of support policies, from many parties to the shortage of drug supply guarantee to give the necessary support. Development and reform (price), and other departments to deepen the reform of the drug prices, strengthen price behavior oversight, improve the price monitoring and early warning system, combined with the existing capital channel on the related monitoring and early warning information system and supply business collaborative application platform construction as a whole. The industrial and information departments should actively guide the production enterprises of the shortage to carry out technological transformation and improve the production and supply capacity. The medical insurance management department should improve the management measures of medical insurance, and make sure that the drug payment is guaranteed. The business department should encourage drug distribution enterprises to develop modern drug distribution, optimize the distribution network of logistics and improve the accessibility of medicines. Family planning department of public health, national drug policy system should be improved and the optimization of drug purchase mechanism, strengthen the drug quality decline caused by malignant price competition and shortage of risk assessment, to carry out the shortage of drug procurement system directly netting, strengthen the shortage of medical institutions and drug with priority, as a whole to adjust and use of supervision, to ensure that procurement specifications, delivery timely and reasonable use, ensure supply. Sasac needs to give full play to the role of state-owned enterprises and promote state-owned enterprises to better fulfill their social responsibilities in the supply of shortage medicines. Shortage of food and drug regulators to strengthen drug quality supervision, perfect the shortage of active pharmaceutical ingredients and drug registration approval policies, the shortage of clinical need give priority review for examination and approval, active pharmaceutical ingredients and drug shortage of study drug list in production of active pharmaceutical ingredients and drug registration system, to ensure the safety of drug quality.
Strengthening policy publicity and popularization of science. Insist the right public opinion direction, through a variety of forms such as television, radio, newspaper, network, shortage of widely publicized the significance of drug supply security and the major policies and measures, targeted to do a good job of popular science shortage of drugs for clinical use. Public related information in time, in proper form to report to the public on a regular basis, to actively respond to and properly handle the drug shortage of public attention, for the enterprise research and development production, medicine circulation development direction, clinical medicine and other fields, the allocation of resources, and provide the important reference of policy making, to guide the healthy, standard and sustainable development of industry.
Annex: a list of key tasks for shortage of drug supply
National health and family planning commission national development and reform commission, ministry of industry and information technology
Ministry of human resources social security department of the ministry of commerce
The state council for industry and commerce, the state administration for industry and commerce, the general administration of food and drug administration
June 27, 2017
The attachment
The shortage of the supply of medicines to ensure the priority of the division of labor
The serial number
Key tasks
The responsibility department
Time limit to complete
one
Establish and improve national drug supply shortage of security work in consultation with the linkage mechanism, working rules for national and provincial levels as a whole and departments responsibility division of labor, to guide the provincial drug supply shortage of security work in consultation with the linkage mechanism construction.
Lead unit: national health and family planning commission
Participating departments: national development and reform commission, ministry of industry and information technology, ministry of finance, human resources social security department, ministry of commerce, state-owned assets supervision and administration commission of the state council, general administration of industry and commerce, food and drug administration
By the end of August 2017, the mechanism will be established and sustained
2
We will monitor the sentinel sites throughout the country and rationally lay out shortages of medicines, and formulate monitoring procedures, scope and time-limits, and implement the monthly zero reporting system for monitoring information. To establish the national, provincial, prefectural and county level monitoring network system and early warning mechanism.
Lead unit: national health and family planning commission
Participating departments: ministry of industry and information technology, ministry of commerce, state council of state council of state council, food and drug administration
Finish the sentinel layout by the end of December 2017; We will continue to build an early warning mechanism
3
Summarize the monitoring information, formulate and regularly publish the list of shortage drugs, through the comprehensive evaluation of drug clinical, dynamic optimization and adjustment; To construct the multi-source information acquisition and supply business cooperation platform of the national and provincial level shortage drugs.
Lead unit: national health and family planning commission
Participating departments: national development and reform commission, ministry of industry and information technology, sasac, food and drug administration
Start work in June 2017; A list of the first shortages by the end of November 2017; In 2018, we will basically complete the application platform construction
4
Master production distribution enterprises and medical institutions the actual inventory, promote clinical demand side and supply side information for producing and distributing strengthen docking, timely find shortage, timely consultation dispensing drugs.
Leading unit: national health and family planning commission
Participating departments: national development and reform commission, ministry of industry and information technology, ministry of commerce, state-owned assets supervision and administration commission of the state council, food and drug administration
By the end of December 2017, the working plan and the basic information collection mechanism will be formulated. The ongoing
5
Organize relevant departments and enterprises, through the price negotiations, the market set a variety of ways, such as to determine a reasonable purchase price, straighten out the market transaction price formation mechanism, to ensure steady supply of drugs.
Lead unit: national health and family planning commission
Participating departments: national development and reform commission, ministry of industry and information technology, food and drug administration
The ongoing
6
To improve drug centralized purchasing and equipped with use, and optimization of drug purchase mechanism, strengthen the drug quality decline caused by malignant price competition and shortage of risk assessment, to carry out the shortage of drug procurement system directly netting, strengthen the shortage of medical institutions and drug with priority, as a whole to adjust and use of supervision, strict enforcement of credit records and repel the market system, to ensure that procurement specifications, delivery timely and reasonable use, ensure supply.
Lead unit: national health and family planning commission
Participating departments: national development and reform commission, ministry of industry and information technology, ministry of commerce, food and drug administration