中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
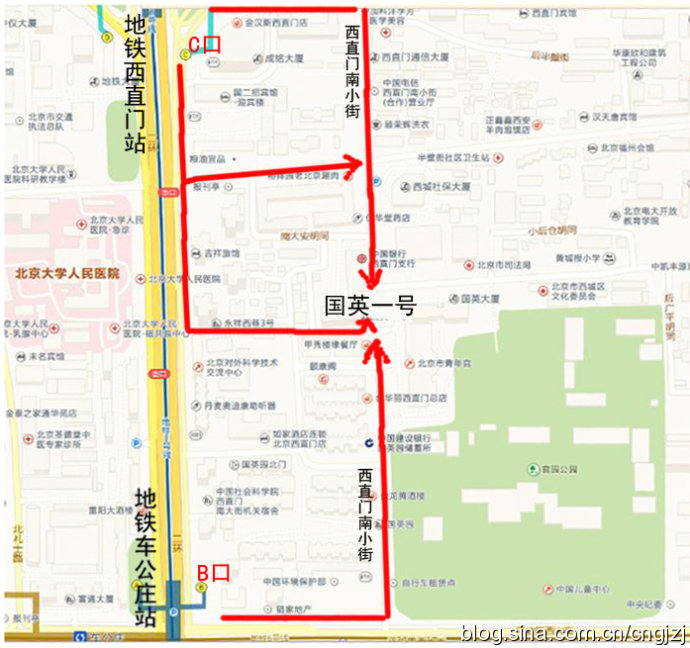
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
Regulations >>
Regulations
您现在的位置是:
首页 >>
Regulations >>
Regulations
2017年08月03日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
Interpretation of traditional Chinese medicine (24)
Time: 2017-07-21
Source: Chinese medical journal
Article 24 the pharmaceutical supervisory and administrative departments under the state council shall organize and strengthen the monitoring of the quality of Chinese medicinal materials and publish the monitoring results regularly to the community. The relevant departments under the state council shall assist in the quality monitoring of Chinese medicinal materials.
The collection, storage of Chinese medicinal materials and the preliminary processing of Chinese medicinal materials shall conform to the relevant technical standards, standards and regulations of the state.
The state encourages the development of the modern circulation system of Chinese medicinal materials, improves the technical level of Chinese medicinal materials packaging and storage, and establishes a traceability system for Chinese medicinal materials. Drug manufacturers shall establish a system of inspection records for the purchase of Chinese medicinal materials. The operator of Chinese medicinal materials shall establish a system for the inspection and purchase and sale of goods, and indicate the origin of Chinese medicinal materials.
This article is about the quality monitoring of Chinese medicinal materials and the construction of Chinese medicinal materials.
At present, the market management level and equipment facilities of Chinese medicinal materials are relatively backward, and there are some problems such as chaotic transaction, lack of quality guarantee and lack of management. To solve these problems, on the one hand, to strengthen supervision, on the one hand, to the traditional backward circulation pattern change, from the collection, distribution of the medicinal materials of organization change, warehousing logistics, packaging, etc. From recently published a series of files and you can see, the relevant state departments are taking steps to promote the establishment of Chinese herbal medicine production, circulation and use of link quality safety guarantee system, including the Chinese modern circulation system. "Strategic plan for development of Chinese medicine (2016-2030)", build a modern Chinese herbal medicine circulation system, to develop Chinese herbal medicine circulation system construction plan, construction of a batch of Chinese standardized, intensive, large-scale and traceability of raw and warehousing logistics center, supplier management and quality traceability system with production enterprises are closely linked. Establish the whole process quality management and quality traceability system of Chinese medicinal materials.
This article is divided into three categories, mainly including the following aspects:
One is the quality monitoring of Chinese herbal medicine. Chinese medicine is also a part of the drug. According to the division of responsibilities, the drug administration department has the responsibility to supervise the production, operation and quality of medicines. The quality of Chinese medicinal materials is related to the quality of Chinese medicine tablets and preparations, and the monitoring system for its quality can be detected in time, so as to avoid unqualified Chinese medicinal materials into the market and cause harm. The monitoring results should be published regularly to the public, so that the public can master the quality information of Chinese herbal medicines at any time. Chinese medicine management involves multiple departments, in addition to the agency, also involved in the department of agriculture, the labor letter department, forestry department and so on, in the process of implementation of monitoring, needed by the drug supervision departments and other relevant departments according to the division of responsibilities, establish coordination mechanism, to work together in the traditional Chinese medicinal materials quality source this way.
The second is the acquisition, storage and preliminary processing of Chinese medicinal materials. The second paragraph of this article stipulates that the collection, storage of Chinese medicinal materials and the preliminary processing of Chinese medicinal materials shall conform to the relevant technical standards, standards and regulations of the state. Traditional Chinese medicinal materials in the collection, storage preservation and maintenance in the process of the processing and easy to eat by moth, mildew to change, the phenomenon such as volatile, weathered metamorphic, so the storage of the herbs and custody should be managed according to standards and specifications. "Chinese herbal medicine production quality management standard (trial)" collection, storage, processing of medicinal herbs such as link requirements made regulations, such as medicinal herbs collected according to the product quality and plant or animal farming quantity capacity per unit area, and refer to the factors such as traditional harvest experience to determine the appropriate recovery time (including the harvest time, collecting fixed number of year), and methods. After the collection of medicinal parts, after selection, cleaning, cutting or dressing, the Chinese medicinal materials should be free from pollution and the effective ingredients will not be destroyed. In the application of traditional storage methods, new technology and equipment of modern storage and storage should be chosen.
Third, the Chinese medicinal materials modern circulation system construction. In 2014, the ministry of commerce issued a notice on the development of the modern logistics system of Chinese medicinal materials, and guided the establishment of the modern logistics system of Chinese medicinal materials. In 2015, the ministry, the state bureau of traditional Chinese medicine and other departments jointly issued the protected traditional Chinese medicinal materials and development planning (2015-2020) ", build a modern circulation system of Chinese medicinal materials, perfect the Chinese herbal medicine circulation industry norms, the construction of modern logistics system of traditional Chinese medicine, promote the development of Chinese herbal medicine circulation system standardization and modernization. In 2016 the strategic plan for development of Chinese medicine (2016-2030) "is put forward, to develop Chinese herbal medicine circulation system construction plan, construction of a batch of Chinese standardized, intensive, large-scale and traceability of raw and warehousing logistics center, supplier management and quality traceability system with production enterprises are closely linked. Developing Chinese medicinal materials e-commerce. Using big data to strengthen the production information collection, price dynamic monitoring analysis and forecast warning. Implement the quality assurance project of Chinese medicinal materials, establish the whole process quality management and quality traceability system of Chinese medicinal materials, and strengthen the construction of third-party testing platform. Modern circulation system, establishing and perfecting the Chinese medicinal materials, the realization of the whole industry chain of quality safety traceability and efficient security system, for the sustained and healthy development of the traditional Chinese medicine (TCM) industry in China has important strategic significance.
The fourth is to establish the specific requirements for the traceability system of Chinese medicinal materials. The establishment of Chinese medicinal materials traceability system is the duty of Chinese herbal medicine producers. The production operator shall implement the main responsibility. In paragraph 3, buy Chinese herbal medicine Chinese herbal medicine production enterprises shall establish incoming inspection record system, Chinese herbal medicine business operator shall establish the system of inspection and purchasing records of goods, and indicate the origin of traditional Chinese medicine. Incoming inspection and purchasing records system, is to establish the specific means of tracing system, is advantageous to the medicinal materials traceability, can realize Chinese herbal medicine "origin can be traced back, go back, can check the quality, responsibility to investigate", from the origin to the market to use the whole terminal chain quality control.
(excerpts from the interpretation of Chinese medicine law of the People's Republic of China, China democratic legal press)