中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
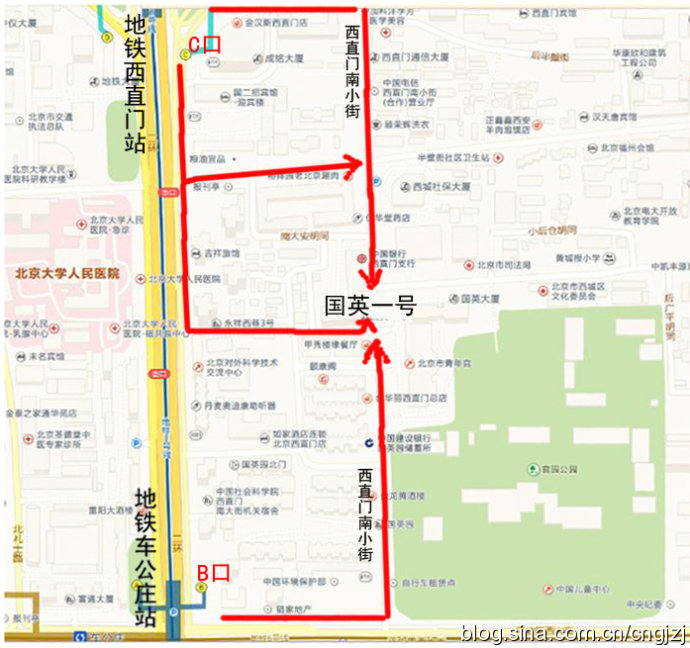
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
Regulations >>
Regulations
您现在的位置是:
首页 >>
Regulations >>
Regulations
2017年09月05日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
About print and distribute to correct unhealthy tendencies in pharmaceutical sales and medical
services in 2017 special governance work points to notify the release time: the 2017-08-28
provinces, autonomous regions and municipalities directly under the central government health and family planning commission, national development and reform commission, price bureau, the competent department of industry and information technology, financial department (bureau), human resources, social security department (bureau), the department (bureau) of commerce, state administration of taxation, industrial and commercial bureau, food and drug supervision bureau, the xinjiang production and construction corps, price bureau, health bureau, national development and reform commission of industry and information technology commission, bureau of finance, human resources and social security bureau, food and drug supervision bureau:
Now will be the correct unhealthy tendencies in pharmaceutical sales and medical services in 2017 special projects work key point of printed and distributed to you, please combined with the actual, carefully carried out.
The national health and family planning commission of the national development and reform commission
The ministry of industry and information technology ministry of finance
Human resources social security ministry of commerce
Total bureau of wu of tax of the state administration for industry and commerce
The food and drug supervision bureau
On July 11, 2017
Correct pharmaceutical sales and medical services in 2017
Rampant special projects work points
Seven times to seriously implement the 18th session of the central commission for discipline inspection plenum spirit, in accordance with the \"government work report in 2017\" and the fifth honest work meeting the relevant requirements of the state council, surrounding the deepening medical and health system reform of the big picture and use policy \"on further reform and improve the pharmaceutical production circulation of several opinions of the implementation, strengthen the supervision and administration of pharmaceutical sales field, serious medical service industry discipline, focus, vigorously promotes the source, strive to drug distribution to the implementation of the\" two votes \", standardize medical consumables reasonable use, strengthen health supervision and relief funds and to consummate persistent effect mechanism to report progress and results.
A \"two votes\", and carry out drug distribution to increase efforts to deal with problems
(a) to promote the \"two votes\", we will improve the mechanism of drug purchase.With comprehensive reform pilot provinces (autonomous regions and municipalities) and public hospital reform pilot city, across the country for drug distribution \"two votes\".To combine the actual, the perfect specific measures, take effective measures to supervise the drug distribution enterprises and medical institutions to establish complete records of purchased pharmaceuticals.Issued as prescribed in the process of drug distribution, related documents, notes, books, goods, invoice and payment for goods.Encourage conditional area to explore in the field of drug distribution to implement the standardization of the paper and electronic.In accordance with the principle of transparency, fair competition, science set up the evaluation factors, increase participation in the drug centralized purchasing of medical institutions, to further the implementation of drug classification procurement policy.Encourage conditional area to explore and joint procurement specialist hospital.Payment in fully implementing health care reform or established medicare drug payment standard, should be allowed to public hospital in the provincial drug centralized purchasing platform or provincial public resources trading platform combined with quantity, purchasing budget.To strengthen the construction of the standardization of the provincial drug centralized purchasing platform, improve the data sharing mechanism, to strengthen the supervision and management of centralized purchasing various participants, eliminate illegal violations of hidden dangers.
(2) of regulation problems, strengthen the industry regulation.Press for rectification work in the field of drug circulation, should be continued to crack down on loan business licenses, false trading, falsified records, illegal channels selling drugs, commercial bribery, fraud, price monopoly price and forge or falsely making out and other illegal ACTS related to invoices, accountable those responsible;Strictly implement the system of credit records, to further improve relevant laws and regulations and establish a drug procurement agencies (drug centralized purchasing platform) as the main body, all relevant departments involved in the credit information of record, communication, public mechanism;To verify the violation behavior, included in the drug purchase record, enterprises credit and personal credit records, in accordance with the public to participate in bidding qualification information of enterprises and institutions and credit history.Clues about crime should be transferred to judicial organs to deal with, or if the circumstances are serious for the recidivist, heavier punishment according to law, improve the violation cost.Strengthen the management of medical representative, standardize medical representative practice, explore the build system of medical representative register put on record, to a medical representative in violation of regulations, engaged in drug sales behavior, shall be in accordance with the relevant regulations, serious processing.
Second, standardize medical consumables supply, increase the supervision over the use of reasonable
(a) standard supply behavior, strengthening.Regions to do management responsibility of the institutions and personnel to carry out the implementing, the work carries out, with the disposable infusion, disposable catheters (bag), vascular/non vascular interventional, pacemakers, orthopaedic implants, dental materials, stapling, etc as the key point, check the standardize production, supply, ensure the security of the common use of medical consumables.Makes some regulation in the field of medical consumable materials in public bidding, to strictly implement medical consumables for mining rules as the focus of the rectification.Supervision in the government-led, throughout the provinces (autonomous regions and municipalities) as the unit of high value medical consumables online centralized purchasing work, check the provinces (autonomous regions and municipalities) medical consumables centralized procurement process construction, system construction and the relevant supporting construction, supervision national drug (consumables) supply security integrated management information platform construction, increase of relevant institutions in violation of high value medical consumables penalties of centralized purchasing behavior.Used to strengthen medical consumables and equipment procurement behavior of supervision and inspection, seriously investigate under the guise of leasing, donations, put in the equipment form, binding supplies and equipment sales of commercial bribery ACTS of unfair competition.
(2) in order to strengthen the management of medical consumables, raise the level of rational use.Promote the medical consumables information publicly, will be the main medical consumables into active disclosure scope, the requirement of medical institutions open consumable price, provide relevant cost query service to patients, improve the transparency of the medical expenses.Reinforcement of high value medical consumables, especially plant involved in the price regulation of type of medical consumables, strictly implement the core system of medical quality and medical safety, strengthen the supervision of medical technology and ensure the quality of medical security.Strengthen clinical rational use of medical equipment and safety management, normative management of medical consumables of generic, for medical consumables usage, dynamic monitoring, to carry out medical consumables quality evaluation.Extensive enhancement activity, and constantly improve the public satisfaction.For medical personnel in violation of the provisions of \"nine\" is not allowed the behavior investigation dynamics, such as serious medical institutions to hold to the problem of the responsibility of leadership.
Third, to strengthen the construction of the industry as a whole style, jointly promote health supervision level
\"Nine\" is not allowed to carry out medical and health enhancement of construction regulations, regulating the behavior of diagnosis and treatment, the \"three reasonable\" (rational drug use, reasonable inspection, diagnosis and treatment), as the key medical insurance fixed-point access standard strictly, improve the exit mechanism.Perfect health care service agreement management, medical expenses and medical treatment should be brought into the regulatory focus.A crackdown on \"insurance fraud cheat to help\" and so on the infringement of the interests of the violation behavior.For new type of rural cooperative medical care and basic medical insurance for urban residents subsidy funds, such as the medical subsidy funds supervision and inspections, investigate intercept, squeezed occupy, divert, false collecting and defraud or defraud money to help others.The popularization and application of increased health intelligent monitoring system, improve the ability of real-time monitoring.
Fourth, to strengthen the construction of department of linkage mechanism, increase the breadth and depth of treatment
All departments to coordinate report of information sharing platform, set up major cases, major orientation problem zone spreading mechanism, form the work report of powerful force.Strengthen efforts to deal with problems in medical service field, all departments should control their respective responsibilities in accordance with the law seriously punish illegal enterprises and medical organizations, serious shall be investigated for responsibility of the person in charge of related and bad credit records, raise the cost of the violation.To strengthen the supervision of private medical institutions, from the planning and construction of private medical institutions, the integration of private medical institutions, professional engineer license approval process, such as regulating the admittance of civilian battalion hospital, create conditions for civilian battalion hospital specification operation and healthy development.Guide the industry play a role of self-purification constraints, strengthen the industry regulation, to establish exit mechanism of medical institutions and their employees.Strengthen those who participate in medical institutions and private credit system construction, and promote the healthy atmosphere, improve the good faith consciousness.
All localities and departments must, in accordance with the \"who supervises, who is responsible for\" and the principle of \"pipe industry must tube enhancement\", stick to work points, according to a close collaboration.Through special projects to improve work report of the institutionalized and standardized and procedural level.To the report of work as a top priority, overall planning, comprehensive deployment, chi and persistently stick to grasp business catch report for a long time for work, task decomposition, detailed assessment indicators, strengthen supervision and inspection, to ensure that the work carries out.Joint inter-ministerial meeting will work to focus on the supervision inspection, for lack of rite, enforcement on loose parts and units;To have no, YouJinBuZhi, against violations of the typical cases to investigate, and accountability of those responsible.